Introduction
In accordance with data from the World Health Organization (WHO), diseases associated
with obesity have become one of the main health problems worldwide. The number of
overweight people in almost every region of the world (except in certain sub-Saharan
African regions and some Asian areas) has been increasing at a constant annual rate
of 0.7% since 1975 to the end of the second decade of the 21st century
(World Health Organization, 2018) Using
the body mass index (BMI) scale, the WHO pointed out that in 2016, more than 39% of
people older than 18 years old (more than 1,900 million) were overweight, while 13%
of the world’s population (more than 650 million people) was diagnosed with obesity.
Among children and teenagers within the age interval of 5-19 years and children
under 5 years old, 18% (over 340 million) and 6% (more than 113 million children)
were overweight, respectively (Murray, 2019;
Pearlman, Obert & Casey, 2017; Stanhope, 2016) . This worldwide phenomenon in
which there are more overweight than underweight people was recognized since the
last third of the 20th century, indicating that two out of the three
countries in North America (namely, México and the United States), and many
countries of the European Union, had the most affected population by this health
crisis (Hruby & Hu, 2015; Ogden, Yanovski, Carroll & Flegal, 2007;
Pereira et al., 2020;
Smith & Smith, 2016).
It is generally stated that the main cause of obesity is related to an imbalance
between the calories consumed and the calories expended. In accordance with WHO
experts (World Health Organization, 2018),
obesity problems can be explained considering that “there is an increased
intake of energy-dense foods that are high in fat, along with an increase in
physical inactivity due to the increasingly sedentary nature of many forms of
work, changing modes of transportation, and increasing
urbanization”.
However, to prevent and treat the obesity problem, experts need to clearly understand
lipogenesis and lipolysis, as well as the processes that determine the formation of
adipose tissue derived from both sugar-rich foods, whose main ingredient is
fructose, and foods high in fat (Moran &
Ladenheim, 2016; Priyadarshini &
Anuradha, 2017). In other words, it is essential to understand the
glucose-fatty acid cycle, also known as the Randle cycle, to recognize the causes of
obesity and propose preventive and effective measures (Randle, Garland, Hales & Newsholme, 1963).
Likewise, the general population should be aware of the seventy different names given
to sugar that are included in processed foods, in order to keep track of excessive
carbohydrate consumption (Gómez Candela & Palma
Milla, 2013; Rodríguez Delgado,
2017). It is estimated that sugar-sweetened beverages (soft drinks,
juices, nectars, teas, energy drinks, yogurts, among others) are the main sources of
sugar in the diet, accounting for more than 15% of the daily caloric intake.
Besides, many people do not even realize that their consumption of sugar-sweetened
beverages and low-nutrient density foods is much more frequent than they think
(Jensen et al., 2018;
Rodríguez Delgado, 2017).
This increase in sugar consumption has been associated with pathologies such as liver
steatosis, type 2 diabetes mellitus, simple and combined hyperlipidemias
(hypertriglyceridemia and hypercholesterolemia), cardiovascular diseases
(hypertension, and heart failure) and dental caries, the latter originally described
as the only disorder due to sugar consumption. Therefore, in this review we updated
the information regarding the Randle cycle, proposed in 1963 (Randle et al., 1963), and the balance between
the formation of acylglycerols and their breakdown (lipogenesis/lipolysis).
The Randle cycle and its association with the balance between lipogenesis and
lipolysis
Postprandial state
Under hyperglycemic conditions, such as the postprandial state, insulin induces
an increase in the expression of glycolytic regulatory enzymes (glucokinase;
phosphofructokinase 1, PFK-1; and pyruvate kinase) and the glucose transporter
GLUT 4 (Figure 1). Insulin also activates
genes that code for enzymes involved in the Randle cycle (Table I), leading to an increase in the glycolytic and Krebs
cycle fluxes and the stimulation of anabolic pathways, such as lipogenesis,
β-reduction [synthesis of fatty acids in the cytosol catalyzed by the Fatty Acid
Synthase (FAS)], phospholipogenesis and cholesterogenesis (Marcelino et al., 2013; Nakamura, Yudell & Loor, 2014; Palomer, Salvado, Barroso & Vázquez-Carrera,
2013; Possik, Madiraju & Prentki,
2017).
Figure 1
Metabolic pathways involved in the extended Randle cycle.
Abbreviations: GK: Glucokinase; PFK-1: Phosphofructokinase-1; PK:
Pyruvate Kinase; PDC: Pyruvate Dehydrogenase Complex; PEPCK:
Phosphoenolpyruvate Carboxykinase; PCmt: mitochondrial Pyruvate
Carboxylase; ACC: Acetyl-CoA Carboxylase; HMGCoA reductase:
Hydroxymethylglutaryl-CoA reductase; acyl-ACP: acyl-acyl-carrier
protein; LPL: Lipoprotein Lipase; HSL: Hormone-Sensitive Lipase;
CAT1: Carnitine Acyltransferase 1; Chol: Cholesterol; TAG:
Triacylglycerol; DAG: Diacylglycerol; FABP: Fatty Acid Binding
Protein; FATP: Fatty Acid Transporter Protein; FAT/CD36: Fatty Acid
Transporter. Enzymes and pathways stimulated by insulin are
highlighted in black; enzymes and pathways activated by glucagon and
norepinephrine are highlighted in blue. Black boxes without color
frames indicate enzymes whose overexpression increases in the
postprandial state; blue boxes indicate enzymes up-regulated by
fasting (glucagon and epinephrine). Black boxes with yellow frames
indicate the main pathways promoted in the postprandial state. Blue
boxes with a green frame highlight the main pathways activated
during hypoglycemia resulting from fasting. * Reactions that take
place in the mitochondrial matrix. Modified from Nelson & Cox, 2017; Aguilar et al.
2017.
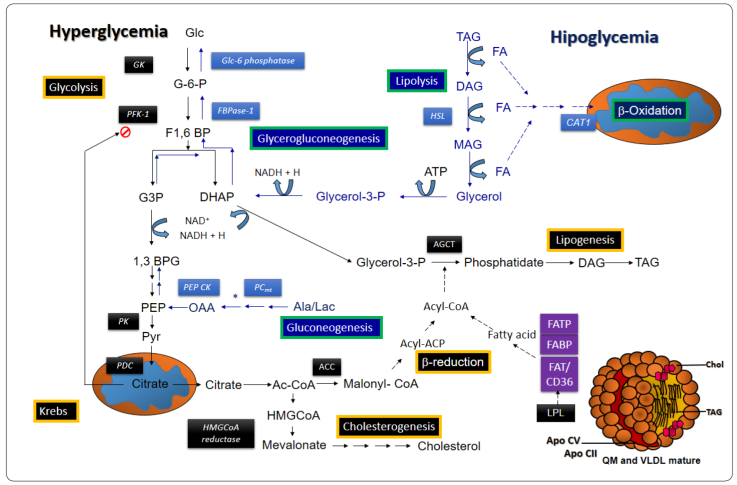
Table I
Enzymes involved in the Randle cycle. Insulin increases the entry
of glucose into the cells, the rate of glycolysis, the pentose
phosphate pathway, as well as some anabolic pathways that are fed by
the carbon skeletons derived from glucose. Some examples concerning
these pathways are β-reduction and lipogenesis. Modified from Nelson & Cox, 2017.
|
Increased expression |
Metabolic
pathway |
|
Hexokinase II |
Glycolysis |
|
Hexokinase IV |
Glycolysis |
|
Phosphofructokinase-1 |
Glycolysis |
|
Pyruvate kinase |
Glycolysis |
|
Phosphofructokinase-2/Fructose-2,6-bisphosphatase |
Glycolysis/gluconeogenesis regulation |
|
Glucose 6-phosphate dehydrogenase |
Phosphopentose
pathway |
|
6-phosphogluconate dehydrogenase |
Phosphopentose
pathway |
|
Pyruvate dehydrogenase complex |
Krebs cycle
entry |
|
Acetil-CoA carboxylase |
β-reduction |
|
Malic enzyme |
β-reduction |
|
Citrate lyase cytosolic |
β-reduction |
|
Fatty acid synthase |
β-reduction |
|
Acyl-CoA-glycerol transferase |
Lipogenesis |
|
Decreased expression
|
Metabolic pathway
|
|
Phosphoenolpyruvate carboxykinase |
Gluconeogenesis |
|
Glucose 6-phosphatase |
Glycemic
regulation |
In terms of metabolic pathways, it can be inferred that a sugar overload in
glycolysis will drive some of the glucose carbons towards dihydroxyacetone
phosphate (DHAP) (Figure 1), which is
involved in the formation of acylglycerols (lipogenesis) and phospholipids
(phospholipogenesis) (Song, Xiaoli & Yang,
2018) (Figure 1). Therefore,
glycolytic flux and anaplerotic pathways are activated in the presence of
insulin (Ameer, Scandiuzzi, Hasnain, Kalbacher,
& Zaidi, 2014; Bartelt et
al., 2013; Summermatter
et al., 2009).
Carbon overload in glycolysis is also associated with the transfer of citrate
from mitochondria to the cytosol, where oxaloacetate (OAA) and acetyl-CoA are
produced by the ATP citrate lyase (Figure
1). The first of these metabolites can be reduced or transaminated and
returned to the mitochondrial matrix, forming part of the malate-aspartate
shuttle.
Acetyl-CoA can take two pathways in the cytosol: the formation of fatty acids or
the synthesis of cholesterol (Figure 1).
Fatty acid formation is controlled by the FAS and the presence of allosteric
regulators of the acetyl-CoA carboxylase (ACC) (Figure 1). In the presence of insulin, β-oxidation (the
mitochondrial catabolic process of breaking down fatty acids) is inhibited by
malonyl-CoA, stopping the transport of fatty acids into the mitochondrial matrix
mediated by the fatty acid transporter Carnitine Acyltransferase 1 (CAT1) (Figure 1).
Regarding cholesterol formation, the pathway is regulated by the
hydroxymethylglutaryl-CoA reductase (HMG-CoA reductase). The fate of
acetyl-CoA’s carbons can be defined as tissue-dependent, and regulated by the
formation of malonyl-CoA and mevalonate metabolites, which control the rate of
β-reduction and cholesterogenesis, respectively (Barbosa & Siniossoglou, 2017; Kory, Farese Jr. & Walther, 2016; Mottillo et al., 2014; Rambold, Cohen & Lippincott-Schwartz,
2015).
Insulin also increases the expression of lipoprotein lipase (LPL) in the
postprandial state. This allows the hydrolysis of plasma triacylglycerol (TAG)
from exogenous sources (diet), found in chylomicrons, and endogenous sources
(hepatic), present in the Very Low Density Lipoproteins (VLDL) (Figure 1). Proper LPL functioning is
associated with adapter proteins that stabilize and activate LPL (Quiroga & Lehner, 2012), such as
apoprotein C-II on the smooth and skeletal muscles, and adipose tissue (Figure 1). Also, hydrolysis of TAG is more
efficient when apoprotein C-V is active.
Fasting conditions
Under fasting or starvation conditions, lipolysis in white adipocytes is
increased by hormones, such as glucagon (Pereira
et al., 2020) and norepinephrine, which activate
the hormone-sensitive lipase (Figure 1),
and decrease the activity of the enzymes that control lipid anabolism, such as
HMG-CoA reductase, ACC, and LPL (Hilton, Karpe
& Pinnick, 2015; Quiroga &
Lehner, 2018; Rambold et
al., 2015). Due to their hydrophobic character, free
fatty acids exported to the blood plasma are transported by albumin toward the
muscle and liver tissues. Uptake of fatty acids into the liver or muscle cells
is carried out by Fatty Acid Binding Protein (FABP), Fatty Acid Transporter
Protein (FATP), and Fatty Acid Transporter (FAT-CD36) (Figure 1). Intracellular fatty acids are then activated in
the form of acyl-CoA in hepatocytes and muscle cells and subsequently
translocated into the mitochondrial matrix by the fatty acid transporter CAT1
and degraded by β-oxidation (Figure 1).
Acetyl-CoA overproduction by β-oxidation of fatty acids causes the allosteric
inhibition of the pyruvate dehydrogenase complex (Figure 1). This allows the production of OAA from pyruvate, and thus
the beginning of hepatic gluconeogenesis (Figure
1) (Fuchs et al.,
2012; Sánchez-Gurmaches et
al., 2018). Glycerol obtained from TAG degradation is
incorporated at the level of DHAP, feeding the gluconeogenesis in the liver
(Figure 1). Glycerol is the most
efficient gluconeogenic substrate, compared to alanine, lactate, and other
carbon skeletons of some gluconeogenic amino acids (Figure 1). In energy terms, the synthesis of one molecule of
glucose from glycerol requires two ATP molecules, instead of six ATP equivalents
if gluconeogenesis begins from pyruvate (Fry
& Carter, 2019; Pietrocola
et al., 2017).
Hepatic metabolism of fructose
Fructose, obtained from fruits and honey, is an intense-flavor sweetener that is
added to most processed foods (Bray, 2013;
Feinman & Fine, 2013). Fructose
presentations include free fructose, sucrose, polysaccharides (fructans) in syrups
and nectars, among others (Choo et
al., 2018). The rise in fructose consumption has been
associated with the increase in obesity and the onset of the metabolic syndrome
(Elliott, Keim, Stern, Teff & Havel,
2002; Sievenpiper et
al., 2014) (Figure 2). This
type of sugar is metabolized largely by hepatocytes, and its assimilation takes
place in parallel with the catabolism of other hexoses in glycolysis (Ter Horst & Serlie, 2017). Glut 2 mediates
the transport of fructose into the hepatocytes, and the monosaccharide is
phosphorylated by fructokinase C, also known as ketohexokinase. Glyceraldehyde and
DHAP are produced from fructose 1-phosphate by aldolase B, which allows the
integration of fructose into the middle part of glycolysis (Figure 2).
Figure 2
Metabolic pathways involved in the assimilation of fructose.
Abbreviations: TAG, Triacylglycerol; DAG, Diacylglycerol; MAG,
Monoacylglycerol; IMP; Inosine monophosphate; AMP; Adenosine
monophosphate. Modified from Nelson
& Cox, 2017.
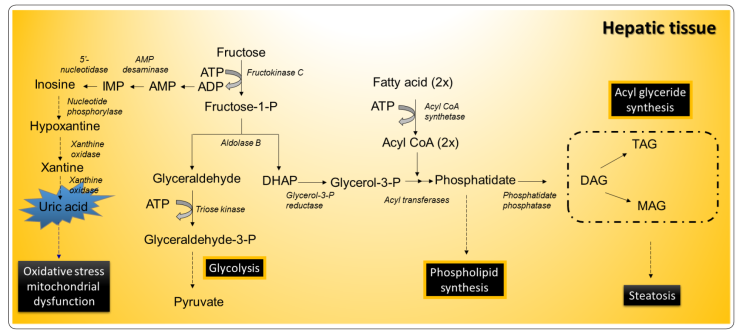
Fructose is a highly lipogenic sugar in comparison with other monosaccharides (Loza-Medrano et al., 2019;
Mai & Yan, 2019), because it enters
the glycolytic pathway without any allosteric or hormonal control of the
fructokinase C. For instance, hexokinases and PFK-1 prevent an accelerated rate of
ATP consumption and avoid the overproduction of ADP and trioses that feed
lipogenesis (Abdelmalek et al.,
2012; Mock, Lateef, Benedito & Tou,
2017).
The increase in the formation of DHAP derived from fructose metabolism, augments the
synthesis of fatty acids and the accumulation of triacylglycerol deposits that can
progress to steatosis (Figure 2), along with an
increase in VLDL and a decrease in High-Density Lipoproteins (HDL) (Ishimoto et al., 2013; Roglans et al., 2007).
At the molecular level, frequent fructose intake increases the production of mRNAs
for FAS and the stearoyl-CoA desaturase 1 (SCD1), which stimulates the synthesis of
triacylglycerols and the introduction of the first double bond to the saturated
fatty acids, respectively (Basaranoglu, Basaranoglu,
Sabuncu & Senturk, 2013). In addition, fructose increases the mRNA of
the Carbohydrate-Responsive Element-Binding Proteins (ChREBP) and the mRNA of
proteins that participate in the STAT3 pathway involved in the release of leptin
(Roglans et al., 2007).
It has been stated that ChREBP is a transcription factor that regulates the
synthesis of enzymes participating in glycolysis, fructolysis and gluconeogenesis.
Also, ChREBP is involved in the de novo synthesis of
triacylglycerols and cholesterol, regardless of insulin activation (Ter Horst & Serlie, 2017).
Frequent fructose intake is associated with hypertension, insulin resistance,
steatosis and hypertriglyceridemia, and causes non-alcoholic fatty liver disease in
people with obesity, in which the nuclear receptor PPARαɣ and its target NF-κβ
participate in the decrease of the rate of β- oxidation under gluconeogenesis
conditions (Costa Gil & Spinedi, 2017;
Laughlin et al., 2014;
Roglans et al., 2002).
High fructose intake is also related to the onset of gout disease (Figure 1). As a consequence of the increase in
fructokinase C activity and the associated high rate of ATP consumption, there is a
rise in the concentrations of ADP and AMP that causes a higher production of uric
acid and inflammation of some joints (Mai & Yan,
2019). The link between fructose intake and gout arthritis has been
observed in various animal models within minutes after the ingestion of fructose
(Jensen et al.,
2018).
In addition, the increase of uric acid levels results in the activation of cytosolic
NADPH oxidase that translocates to the mitochondria, generating oxidative stress and
the inhibition of the aconitase 2, and resulting in the accumulation of citrate in
the mitochondrial matrix (Jamnik et
al., 2016; Jensen et
al., 2018). This causes the export of citrate to the
cytoplasm and the stimulation of lipogenesis and cholesterogenesis (Figure 1). The oxidative stress in mitochondria
spreads to the endoplasmic reticulum, activating the Sterol Regulatory
Element-Binding transcription factor 1 (SREBP-1), which in turn increases the
transcript levels of genes involved in lipogenesis and cholesterol synthesis (Jensen et al., 2018; Lustig, 2010; Samuel, 2011) (Figure 1).
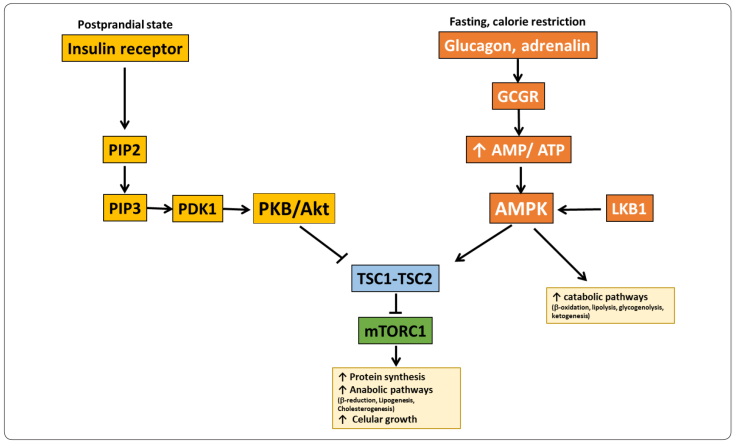
Control of Randle cycle by mTORC1 and AMPK
The mammalian target of rapamycin (mTOR) is a kinase that forms two complexes in
mammals: mTORC1 and mTORC2. mTORC1 is activated by amino acids (Chen, Wei, Liu & Guan, 2014; Cheng & Saltiel, 2006), growth factors and
hormones, such as insulin (Baena et
al., 2015; Verges,
2018). mTORC2 is also regulated by growth factors and is involved in
cytoskeleton remodeling and sphingolipid synthesis (Figure 3). During the postprandial state, insulin stimulates
phosphoinositide-dependent kinase 1 (PDK1), which leads to the activation of PKB/Akt
signaling pathway, inhibition of the TSC1/TSC2 complex (tuberous sclerosis complex 1
and 2), and activation of mTORC1, which promotes lipogenesis, glycolysis, and
glycogen synthesis (Asati, Mahapatra & Bharti,
2016; Jiang et al.,
2008; Kumar et al.,
2010; Naito, Kuma & Mizushima,
2013; Verges, 2018). On the
contrary, the AMP-dependent Kinase (AMPK) is hormonally downregulated under the
hyperglycemia status and activated during fasting or exercise conditions. Activation
of the AMPK depends on the stimulation of both the AMPc-dependent protein kinase
(PKA) and the human tumor suppressor liver kinase 1 (LKB1), and the increase in the
concentration of AMP (Kim & He, 2013).
Along with the stimulation of PKA and AMPK there is a decrease in the main lipogenic
pathways, such as fatty acid synthesis, triacylglycerol accumulation and
cholesterogenesis, and activation of gluconeogenesis (Hasenour et al., 2017), glycogen degradation,
lipolysis and mitochondrial β-oxidation, thereby increasing ketogenesis in the liver
(Cardaci, Filomeni, & Ciriolo, 2012).
AMPK, through the phosphorylation of ACC and HMG-CoA reductase, inhibits the
synthesis of fatty acids and cholesterol, respectively.
Figure 3
Functional relationships between mTORC1 and the AMP activated protein
kinase (AMPK) in the Randle cycle. Abbreviations: AMPK: AMP-Activated
Protein Kinase; mTORC1: mammalian Target of Rapamycin Complex 1; PIP2:
phosphatidylinositol (4,5)-bisphosphate; PI3K: phosphoinositide 3
kinase; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; PDK1:
3-phosphoinositide-dependent kinase-1; PKB/Akt: protein kinase B/Akt;
TSC1-TSC2: 1-2 tuberous sclerosis complex (or hamartin-tuberin complex);
PKA: Protein Kinase A; LKB1: Liver Kinase B1. Modified from Yoon, 2017.
In short, triacylglycerol accumulation in white fat deposits, liver tissue, and
between fiber bundles is caused by hypercaloric diets rich in fast-digesting
carbohydrates, along with the sedentary lifestyle habits of Western societies (Perera & Turner, 2016).
Hypertriglyceridemia and hypercholesterolemia are involved in the pathophysiology of
health problems, such as high blood pressure, diabetes mellitus 2, atherosclerosis
and obesity, among other diseases (Ke, Xu, Li, Luo
& Huang, 2018; Nakamura et
al., 2014; Palomer
et al., 2013; Possik
et al., 2017).
Conclusions
There is a metabolic relationship between sugar consumption and fat accumulation. In
the specific case of fructose, the excessive consumption of this sugar causes
depletion of cellular ATP, steatosis, obesity, metabolic syndrome, and an increase
in the production of uric acid. These adverse metabolic effects are the consequence
of the lack of regulatory mechanisms for the incorporation of fructose into the
glycolytic pathway. A new addition to the Randle cycle is the incorporation of
mTORC1 and the antagonistic effect of the AMPK to ensure an efficient regulation of
lipogenesis and lipolysis, respectively. In terms of public policy, authorities of
health institutes should advise against the abuse of carbohydrate consumption.
Acknowledgments
This work was supported by the Universidad Nacional Autónoma de México (UNAM),
Programa de Apoyo a Proyectos de Investigación Tecnológica [PAPIIT IN222117];
Consejo Nacional de Ciencia y Tecnología, CONACYT [254904-JPP] and [256520-GGS].
Instituto Politécnico Nacional- Secretaría de Investigación y Posgrado, [IPN-SIP
20190200]. We are grateful to Oscar Iván Luqueño Bocardo for the design of Figure 1.
References
Abdelmalek, M. F, Lazo, M., Horska, A., Bonekamp, S., Lipkin, E. W.,
Balasubramanyam, A., Bantle, J. P., Johnson, R. J., Diehl, A. M. & Clark, J.
M. Fatty Liver Subgroup of Look AHEAD Research Group. (2012). Higher dietary
fructose is associated with impaired hepatic adenosine triphosphate homeostasis
in obese individuals with type 2 diabetes. Hepatology,
56(3), 952-960. DOI: 10.1002/hep.25741
M. F Abdelmalek
M. Lazo
A. Horska
S. Bonekamp
E. W. Lipkin
A. Balasubramanyam
J. P. Bantle
R. J. Johnson
A. M. Diehl
J. M. Clark
Fatty Liver Subgroup of Look AHEAD Research Group
2012Higher dietary fructose is associated with impaired hepatic
adenosine triphosphate homeostasis in obese individuals with type 2
diabetesHepatology56(3)95296010.1002/hep.25741
Aguilar, L. R. , Pardo, J. P., Lomelí, M. M., Bocardo, O. I. L.,
Juárez Oropeza, M. A. & Guerra Sánchez, G. (2017). Lipid droplets
accumulation and other biochemical changes induced in the fungal pathogen
Ustilago maydis under nitrogen-starvation. Arch.
Microbiol., 199(8):1195-1209. DOI:
10.1007/s00203-017-1388-8
L. R. Aguilar
J. P. Pardo
M. M. Lomelí
O. I. L. Bocardo
M. A. Juárez Oropeza
G. Guerra Sánchez
2017Lipid droplets accumulation and other biochemical changes induced
in the fungal pathogen Ustilago maydis under
nitrogen-starvationArch. Microbiol.199(8)1195120910.1007/s00203-017-1388-8
Ameer, F., Scandiuzzi, L., Hasnain, S., Kalbacher, H. & Zaidi,
N. (2014). De novo lipogenesis in health and disease.
Metabolism, 63 (7), 895-902. DOI:
10.1016/j.metabol.2014.04.003
F. Ameer
L. Scandiuzzi
S. Hasnain
H. Kalbacher
N. Zaidi
2014De novo lipogenesis in health and diseaseMetabolism63(7)89590210.1016/j.metabol.2014.04.003
Asati, V., Mahapatra, D. K. & Bharti, S. K. (2016).
PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer
agents: Structural and pharmacological perspectives. Eur. J.
Med. Chem., 109, 314-341. DOI:
10.1016/j.ejmech.2016.01.012
V. Asati
D. K. Mahapatra
S. K. Bharti
2016PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors
as anticancer agents: Structural and pharmacological
perspectivesEur. J. Med. Chem.10931434110.1016/j.ejmech.2016.01.012
Baena, M., Sanguesa, G., Hutter, N., Sánchez, R. M., Roglans, N.,
Laguna, J. C. & Alegret, M. (2015). Fructose supplementation impairs rat
liver autophagy through mTORC activation without inducing endoplasmic reticulum
stress. Biochim. Biophys. Acta, 1851(2), 107-116.
DOI: 10.1016/j.bbalip.2014.11.003
M. Baena
G. Sanguesa
N. Hutter
R. M. Sánchez
N. Roglans
J. C. Laguna
M. Alegret
2015Fructose supplementation impairs rat liver autophagy through
mTORC activation without inducing endoplasmic reticulum
stressBiochim. Biophys. Acta1851(2)10711610.1016/j.bbalip.2014.11.003
Barbosa, A. D. & Siniossoglou, S. (2017). Function of lipid
droplet-organelle interactions in lipid homeostasis. Biochim. Biophys.
Acta Mol. Cell Res., 1864(9), 1459-1468. DOI:
10.1016/j.bbamcr.2017.04.001
A. D. Barbosa
S. Siniossoglou
2017Function of lipid droplet-organelle interactions in lipid
homeostasisBiochim. Biophys. Acta Mol. Cell Res.1864(9)1459146810.1016/j.bbamcr.2017.04.001
Bartelt, A., Weigelt, C., Cherradi, M. L., Niemeier, A., Todter, K.,
Heeren, J. & Scheja, L. (2013). Effects of adipocyte lipoprotein lipase on
de novo lipogenesis and white adipose tissue browning. Biochim Biophys
Acta, 1831(5), 934-942. DOI:
10.1016/j.bbalip.2012.11.011
A. Bartelt
C. Weigelt
M. L. Cherradi
A. Niemeier
K. Todter
J. Heeren
L. Scheja
2013Effects of adipocyte lipoprotein lipase on de novo lipogenesis
and white adipose tissue browningBiochim Biophys Acta1831(5)93494210.1016/j.bbalip.2012.11.011
Basaranoglu, M., Basaranoglu, G., Sabuncu, T. & Senturk, H.
(2013). Fructose as a key player in the development of fatty liver disease.
World J. Gastroenterol., 19(8), 1166-1172.
DOI: 10.3748/wjg.v19.i8.1166
M. Basaranoglu
G. Basaranoglu
T. Sabuncu
H. Senturk
2013Fructose as a key player in the development of fatty liver
diseaseWorld J. Gastroenterol.19(8)1166117210.3748/wjg.v19.i8.1166
Bray, G. A. (2013). Energy and fructose from beverages sweetened
with sugar or high-fructose corn syrup pose a health risk for some people.
Adv. Nutr., 4(2), 220-225. DOI:
10.3945/an.112.002816
G. A. Bray
2013Energy and fructose from beverages sweetened with sugar or
high-fructose corn syrup pose a health risk for some peopleAdv. Nutr.4(2)22022510.3945/an.112.002816
Cardaci, S., Filomeni, G. & Ciriolo, M. R. (2012). Redox
implications of AMPK-mediated signal transduction beyond energetic clues.
J. Cell Sci., 125(Pt 9), 2115-2125. DOI:
10.1242/jcs.095216
S. Cardaci
G. Filomeni
M. R. Ciriolo
2012Redox implications of AMPK-mediated signal transduction beyond
energetic cluesJ. Cell Sci.125(Pt9)2115212510.1242/jcs.095216
Chen, Y., Wei, H., Liu, F. & Guan, J. L. (2014). Hyperactivation
of mammalian target of rapamycin complex 1 (mTORC1) promotes breast cancer
progression through enhancing glucose starvation-induced autophagy and Akt
signaling. J. Biol. Chem., 289(2), 1164-1173. DOI:
10.1074/jbc.M113.526335
Y. Chen
H. Wei
F. Liu
J. L. Guan
2014Hyperactivation of mammalian target of rapamycin complex 1
(mTORC1) promotes breast cancer progression through enhancing glucose
starvation-induced autophagy and Akt signalingJ. Biol. Chem.289(2)1164117310.1074/jbc.M113.526335
Cheng, A. & Saltiel, A. R. (2006). More TORC for the
gluconeogenic engine. Bioessays, 28(3), 231-234.
DOI: 10.1002/bies.20375
A. Cheng
A. R. Saltiel
2006More TORC for the gluconeogenic engineBioessays28(3)23123410.1002/bies.20375
Choo, V. L., Viguiliouk, E., Blanco Mejia, S., Cozma, A. I., Khan,
T.A., Ha, V., Wolever, T. M. S., Leiter, L. A., Vuksan, V., Kendall, C. W. C.,
de Souza, R. J., Jenkins, D. J. A. & Sievenpiper, J. L. (2018). Food sources
of fructose-containing sugars and glycaemic control: systematic review and
meta-analysis of controlled intervention studies. BMJ,
363, k4644. DOI: 10.1136/bmj.k4644
V. L. Choo
E. Viguiliouk
S. Blanco Mejia
A. I. Cozma
T.A. Khan
V. Ha
T. M. S. Wolever
L. A. Leiter
V. Vuksan
C. W. C. Kendall
R. J. de Souza
D. J. A. Jenkins
J. L. Sievenpiper
2018Food sources of fructose-containing sugars and glycaemic control:
systematic review and meta-analysis of controlled intervention
studiesBMJ363k4644k464410.1136/bmj.k4644
Costa Gil, J. E. & Spinedi, E. (2017). La tormentosa relación
entre las grasas y el desarrollo de la diabetes mellitus de tipo 2: actualizado.
Parte I. Revista Argentina de Endocrinología y Metabolismo,
54, 109-123. DOI: 10.1016/j.raem.2017.06.001
J. E. Costa Gil
E. Spinedi
2017La tormentosa relación entre las grasas y el desarrollo de la
diabetes mellitus de tipo 2: actualizadoParte IRevista Argentina de Endocrinología y Metabolismo5410912310.1016/j.raem.2017.06.001
Elliott, S. S., Keim, N. L., Stern, J. S., Teff, K. & Havel, P.
J. (2002). Fructose, weight gain, and the insulin resistance syndrome.
Am. J. Clin. Nutr., 76(5), 911-922. DOI:
10.1093/ajcn/76.5.911
S. S. Elliott
N. L. Keim
J. S. Stern
K. Teff
P. J. Havel
2002Fructose, weight gain, and the insulin resistance
syndromeAm. J. Clin. Nutr.76(5)91192210.1093/ajcn/76.5.911
Feinman, R. D. & Fine, E. J. (2013). Fructose in perspective.
Nutr. Metab. (Lond.), 10(1), 45. DOI:
10.1186/1743-7075-10-45
R. D. Feinman
E. J. Fine
2013Fructose in perspectiveNutr. Metab. (Lond.)10(1)454510.1186/1743-7075-10-45
Fry, B. & Carter, J. F. (2019). Stable carbon isotope
diagnostics of mammalian metabolism, a high-resolution isotomics approach using
amino acid carboxyl groups. PLoS One, 14(10),
e0224297. DOI: 10.1371/journal.pone.0224297
B. Fry
J. F. Carter
2019Stable carbon isotope diagnostics of mammalian metabolism, a
high-resolution isotomics approach using amino acid carboxyl
groupsPLoS One14(10)e022429710.1371/journal.pone.0224297
Fuchs, C. D., Claudel, T., Kumari, P., Haemmerle, G., Pollheimer, M.
J., Stojakovic, T., Scharnagl, H., Halilbasic, E., Gumhold, J., Silbert, D.,
Koefeler, H. & Trauner, M. (2012). Absence of adipose triglyceride lipase
protects from hepatic endoplasmic reticulum stress in mice.
Hepatology, 56(1), 270-280. DOI:
10.1002/hep.25601
C. D. Fuchs
T. Claudel
P. Kumari
G. Haemmerle
M. J. Pollheimer
T. Stojakovic
H. Scharnagl
E. Halilbasic
J. Gumhold
D. Silbert
H. Koefeler
M. Trauner
2012Absence of adipose triglyceride lipase protects from hepatic
endoplasmic reticulum stress in miceHepatology56(1)27028010.1002/hep.25601
Gómez Candela, C. & Palma Milla, S. (2013). Una visión global,
actualizada y crítica del papel del azúcar en nuestra alimentación.
Nutrición Hospitalaria, 28,
1-4.
C. Gómez Candela
S. Palma Milla
2013Una visión global, actualizada y crítica del papel del azúcar en
nuestra alimentaciónNutrición Hospitalaria2814
Hasenour, C. M., Ridley, D. E., James, F. D., Hughey, C. C.,
Donahue, E. P., Viollet, B., Foretz, M., Young, J. D. & Wasserman, D. H.
(2017). Liver AMP-Activated Protein Kinase Is Unnecessary for Gluconeogenesis
but Protects Energy State during Nutrient Deprivation. PLoS
One, 12(1), e0170382. DOI:
10.1371/journal.pone.0170382
C. M. Hasenour
D. E. Ridley
F. D. James
C. C. Hughey
E. P. Donahue
B. Viollet
M. Foretz
J. D. Young
D. H. Wasserman
2017Liver AMP-Activated Protein Kinase Is Unnecessary for
Gluconeogenesis but Protects Energy State during Nutrient
DeprivationPLoS One12(1)e017038210.1371/journal.pone.0170382
Hilton, C., Karpe, F. & Pinnick, K. E. (2015). Role of
developmental transcription factors in white, brown and beige adipose tissues.
Biochim. Biophys. Acta, 1851(5), 686-696. DOI:
10.1016/j.bbalip.2015.02.003
C. Hilton
F. Karpe
K. E. Pinnick
2015Role of developmental transcription factors in white, brown and
beige adipose tissuesBiochim. Biophys. Acta1851(5)68669610.1016/j.bbalip.2015.02.003
Hruby, A. & Hu, F. B. (2015). The Epidemiology of Obesity: A Big
Picture. Pharmacoeconomics, 33(7), 673-689. DOI:
10.1007/s40273-014-0243-x
A. Hruby
F. B. Hu
2015The Epidemiology of Obesity: A Big PicturePharmacoeconomics33(7)67368910.1007/s40273-014-0243-x
Ishimoto, T., Lanaspa, M. A., Rivard, C. J., Roncal-Jimenez, C. A.,
Orlicky, D. J., Cicerchi, C., McMahan, R. H., Abdelmalek, M. F., Rosen, H. R.,
Jackman, M. R., MacLean, P. S., Diggle, C. P., Asipu, A., Inaba, S., Kosugi, T.,
Sato, W., Maruyama, S., Sánchez-Lozada, L. G., Sautin, Y.Y ., Hill, J. O.,
Bonthron, D. T. & Johnson, R. J. (2013). High-fat and high-sucrose (western)
diet induces steatohepatitis that is dependent on fructokinase.
Hepatology, 58(5), 1632-1643. DOI:
10.1002/hep.26594
T. Ishimoto
M. A. Lanaspa
C. J. Rivard
C. A. Roncal-Jimenez
D. J. Orlicky
C. Cicerchi
R. H. McMahan
M. F. Abdelmalek
H. R. Rosen
M. R. Jackman
P. S. MacLean
C. P. Diggle
A. Asipu
S. Inaba
T. Kosugi
W. Sato
S. Maruyama
L. G. Sánchez-Lozada
Y.Y . Sautin
J. O. Hill
D. T. Bonthron
R. J. Johnson
2013High-fat and high-sucrose (western) diet induces steatohepatitis
that is dependent on fructokinaseHepatology58(5)1632164310.1002/hep.26594
Jamnik, J., Rehman, S., Blanco Mejia, S., de Souza, R. J, Khan, T.
A., Leiter, L. A., Wolever, T. M., Kendall, C. W., Jenkins, D. J. &
Sievenpiper, J. L. (2016). Fructose intake and risk of gout and hyperuricemia: a
systematic review and meta-analysis of prospective cohort studies. BMJ
Open, 6(10), e013191. DOI:
10.1136/bmjopen-2016-013191
J. Jamnik
S. Rehman
S. Blanco Mejia
R. J de Souza
T. A. Khan
L. A. Leiter
T. M. Wolever
C. W. Kendall
D. J. Jenkins
J. L. Sievenpiper
2016Fructose intake and risk of gout and hyperuricemia: a systematic
review and meta-analysis of prospective cohort studiesBMJ Open6(10)e01319110.1136/bmjopen-2016-013191
Jensen, T., Abdelmalek, M. F., Sullivan, S., Nadeau, K. J., Green,
M., Roncal, C., Nakagawa, T., Kuwabara, M., Sato, Y., Kang, D. H., Tolan, D. R.,
Sanchez-Lozada, L. G., Rosen, H. R, Lanaspa, M. A., Diehl, A. M. & Johnson,
R. J. (2018). Fructose and sugar: A major mediator of non-alcoholic fatty liver
disease. J. Hepatol., 68(5), 1063-1075. DOI:
10.1016/j.jhep.2018.01.019
T. Jensen
M. F. Abdelmalek
S. Sullivan
K. J. Nadeau
M. Green
C. Roncal
T. Nakagawa
M. Kuwabara
Y. Sato
D. H. Kang
D. R. Tolan
L. G. Sanchez-Lozada
H. R Rosen
M. A. Lanaspa
A. M. Diehl
R. J. Johnson
2018Fructose and sugar: A major mediator of non-alcoholic fatty liver
diseaseJ. Hepatol.68(5)1063107510.1016/j.jhep.2018.01.019
Jiang, X., Kenerson, H., Aicher, L., Miyaoka, R., Eary, J., Bissler,
J. & Yeung, R. S. (2008). The tuberous sclerosis complex regulates
trafficking of glucose transporters and glucose uptake. Am. J.
Pathol., 172(6), 1748-1756. DOI:
10.2353/ajpath.2008.070958
X. Jiang
H. Kenerson
L. Aicher
R. Miyaoka
J. Eary
J. Bissler
R. S. Yeung
2008The tuberous sclerosis complex regulates trafficking of glucose
transporters and glucose uptakeAm. J. Pathol.172(6)1748175610.2353/ajpath.2008.070958
Ke, R., Xu, Q., Li, C., Luo, L. & Huang, D. (2018). Mechanisms
of AMPK in the maintenance of ATP balance during energy metabolism. Cell
Biol. In.. 42(4), 384-392. DOI:
10.1002/cbin.10915
R. Ke
Q. Xu
C. Li
L. Luo
D. Huang
2018Mechanisms of AMPK in the maintenance of ATP balance during
energy metabolismCell Biol. In.42(4)38439210.1002/cbin.10915
Kim, I. & He, Y. Y. (2013). Targeting the AMP-Activated Protein
Kinase for Cancer Prevention and Therapy. Front. Oncol.,
3, 175. DOI: 10.3389/fonc.2013.00175
I. Kim
Y. Y. He
2013Targeting the AMP-Activated Protein Kinase for Cancer Prevention
and TherapyFront. Oncol.317517510.3389/fonc.2013.00175
Kory, N., Farese, R. V., Jr. & Walther, T. C. (2016). Targeting
Fat: Mechanisms of Protein Localization to Lipid Droplets. Trends Cell
Biol., 26(7), 535-546. DOI:
10.1016/j.tcb.2016.02.007
N. Kory
R. V. Farese Jr
T. C. Walther
2016Targeting Fat: Mechanisms of Protein Localization to Lipid
DropletsTrends Cell Biol.26(7)53554610.1016/j.tcb.2016.02.007
Kumar, A., Lawrence, J. C. Jr. , Jung, D.Y., Ko, H. J., Keller, S.
R., Kim, J. K., Magnuson, M. A. & Harris, T. E. (2010). Fat cell-specific
ablation of rictor in mice impairs insulin-regulated fat cell and whole-body
glucose and lipid metabolism. Diabetes, 59(6),
1397-1406. DOI: 10.2337/db09-1061
A. Kumar
J. C. Lawrence Jr.
D.Y. Jung
H. J. Ko
S. R. Keller
J. K. Kim
M. A. Magnuson
T. E. Harris
2010Fat cell-specific ablation of rictor in mice impairs
insulin-regulated fat cell and whole-body glucose and lipid
metabolismDiabetes59(6)1397140610.2337/db09-1061
Laughlin, M. R., Bantle, J. P., Havel, P. J., Parks, E., Klurfeld,
D. M., Teff, K. & Maruvada, P. (2014). Clinical research strategies for
fructose metabolism. Adv. Nutr., 5(3), 248-259.
DOI: 10.3945/an.113.005249
M. R. Laughlin
J. P. Bantle
P. J. Havel
E. Parks
D. M. Klurfeld
K. Teff
P. Maruvada
2014Clinical research strategies for fructose
metabolismAdv. Nutr.5(3)24825910.3945/an.113.005249
Loza-Medrano, S. S., Baiza-Gutman, L. A., Manuel-Apolinar, L.,
García-Macedo, R., Damasio-Santana, L., Martínez-Mar, O. A., Sánchez-Becerra, M.
C., Cruz-López, M., Ibáñez-Hernández, M. A. & Díaz-Flores, M. (2019). High
fructose-containing drinking water-induced steatohepatitis in rats is prevented
by the nicotinamide-mediated modulation of redox homeostasis and NADPH-producing
enzymes. Mol. Biol. Rep., 47(1), 337-351. DOI:
10.1007/s11033-019-05136-4
S. S. Loza-Medrano
L. A. Baiza-Gutman
L. Manuel-Apolinar
R. García-Macedo
L. Damasio-Santana
O. A. Martínez-Mar
M. C. Sánchez-Becerra
M. Cruz-López
M. A. Ibáñez-Hernández
M. Díaz-Flores
2019High fructose-containing drinking water-induced steatohepatitis
in rats is prevented by the nicotinamide-mediated modulation of redox
homeostasis and NADPH-producing enzymesMol. Biol. Rep.47(1)33735110.1007/s11033-019-05136-4
Lustig, R. H. (2010). Fructose: metabolic, hedonic, and societal
parallels with ethanol. J. Am. Diet. Assoc.,
110(9), 1307-1321. DOI:
10.1016/j.jada.2010.06.008
R. H. Lustig
2010Fructose: metabolic, hedonic, and societal parallels with
ethanolJ. Am. Diet. Assoc.110(9)1307132110.1016/j.jada.2010.06.008
Mai, B. H. & Yan, L. J. (2019). The negative and detrimental
effects of high fructose on the liver, with special reference to metabolic
disorders. Diabetes Metab. Syndr. Obes., 12,
821-826. DOI: 10.2147/DMSO.S198968
B. H. Mai
L. J. Yan
2019The negative and detrimental effects of high fructose on the
liver, with special reference to metabolic disordersDiabetes Metab. Syndr. Obes.1282182610.2147/DMSO.S198968
Marcelino, H., Veyrat-Durebex, C., Summermatter, S., Sarafian, D.,
Miles-Chan, J., Arsenijevic, D., Zani, F., Montani, J. P., Seydoux, J., Solinas,
G., Rohner-Jeanrenaud, F. & Dulloo, A. G. (2013). A role for adipose tissue
de novo lipogenesis in glucose homeostasis during catch-up growth: a Randle
cycle favoring fat storage. Diabetes, 62(2),
362-372. DOI: 10.2337/db12-0255
H. Marcelino
C. Veyrat-Durebex
S. Summermatter
D. Sarafian
J. Miles-Chan
D. Arsenijevic
F. Zani
J. P. Montani
J. Seydoux
G. Solinas
F. Rohner-Jeanrenaud
A. G. Dulloo
2013A role for adipose tissue de novo lipogenesis in glucose
homeostasis during catch-up growth: a Randle cycle favoring fat
storageDiabetes62(2)36237210.2337/db12-0255
Mock, K., Lateef, S., Benedito, V. A. & Tou, J. C. (2017).
High-fructose corn syrup-55 consumption alters hepatic lipid metabolism and
promotes triglyceride accumulation. J. Nutr. Biochem.,
39, 32-39. DOI: 10.1016/j.jnutbio.2016.09.010
K. Mock
S. Lateef
V. A. Benedito
J. C. Tou
2017High-fructose corn syrup-55 consumption alters hepatic lipid
metabolism and promotes triglyceride accumulationJ. Nutr. Biochem.39,323910.1016/j.jnutbio.2016.09.010
Moran, T. H. & Ladenheim, E. E. (2016). Physiologic and Neural
Controls of Eating. Gastroenterol. Clin. North. Am.,
45(4), 581-599. DOI: 10.1016/j.gtc.2016.07.009
T. H. Moran
E. E. Ladenheim
2016Physiologic and Neural Controls of EatingGastroenterol. Clin. North. Am.45(4)58159910.1016/j.gtc.2016.07.009
Mottillo, E. P., Balasubramanian, P., Lee, Y. H., Weng, C., Kershaw,
E. E. & Granneman, J. G. (2014). Coupling of lipolysis and de novo
lipogenesis in brown, beige, and white adipose tissues during chronic
beta3-adrenergic receptor activation. J. Lipid. Res.,
55(11), 2276-2286. DOI: 10.1194/jlr.M050005
E. P. Mottillo
P. Balasubramanian
Y. H. Lee
C. Weng
E. E. Kershaw
J. G. Granneman
2014Coupling of lipolysis and de novo lipogenesis in brown, beige,
and white adipose tissues during chronic beta3-adrenergic receptor
activationJ. Lipid. Res.55(11)2276228610.1194/jlr.M050005
Murray, R. D. (2019). 100% Fruit Juice in Child and Adolescent
Dietary Patterns. J. Am. Coll. Nutr., 39(2),
122-127. DOI: 10.1080/07315724.2019.1615013
R. D. Murray
2019100% Fruit Juice in Child and Adolescent Dietary
PatternsJ. Am. Coll. Nutr.39(2)12212710.1080/07315724.2019.1615013
Naito, T., Kuma, A. & Mizushima, N. (2013). Differential
contribution of insulin and amino acids to the mTORC1-autophagy pathway in the
liver and muscle. J. Biol. Chem., 288(29),
21074-21081. DOI: 10.1074/jbc.M113.456228
T. Naito
A. Kuma
N. Mizushima
2013Differential contribution of insulin and amino acids to the
mTORC1-autophagy pathway in the liver and muscleJ. Biol. Chem.288(29)210742108110.1074/jbc.M113.456228
Nakamura, M. T., Yudell, B. E. & Loor, J. J. (2014). Regulation
of energy metabolism by long-chain fatty acids. Prog. Lipid.
Res., 53, 124-144. DOI:
10.1016/j.plipres.2013.12.001
M. T. Nakamura
B. E. Yudell
J. J. Loor
2014Regulation of energy metabolism by long-chain fatty
acidsProg. Lipid. Res.53,12414410.1016/j.plipres.2013.12.001
Nelson, D. L. & Cox, M. (2017). Lehninger principles of
biochemistry. W.H. Freeman. New York.
D. L. Nelson
M. Cox
2017Lehninger principles of biochemistryW.H. FreemanNew York
Ogden, C. L., Yanovski, S. Z., Carroll, M. D. & Flegal, K. M.
(2007). The epidemiology of obesity. Gastroenterology, 132(6),
2087-2102. DOI: 10.1053/j.gastro.2007.03.052
C. L. Ogden
S. Z. Yanovski
M. D. Carroll
K. M. Flegal
2007The epidemiology of obesityGastroenterology132(6)2087210210.1053/j.gastro.2007.03.052
Palomer, X., Salvado, L., Barroso, E. & Vazquez-Carrera, M.
(2013). An overview of the crosstalk between inflammatory processes and
metabolic dysregulation during diabetic cardiomyopathy. Int. J.
Cardiol., 168(4), 3160-3172. DOI:
10.1016/j.ijcard.2013.07.150
X. Palomer
L. Salvado
E. Barroso
M. Vazquez-Carrera
2013An overview of the crosstalk between inflammatory processes and
metabolic dysregulation during diabetic cardiomyopathyInt. J. Cardiol.168(4)3160317210.1016/j.ijcard.2013.07.150
Pearlman, M., Obert, J. & Casey, L. (2017). The Association
Between Artificial Sweeteners and Obesity. Curr. Gastroenterol.
Rep., 19(12), 64. DOI:
10.1007/s11894-017-0602-9
M. Pearlman
J. Obert
L. Casey
2017The Association Between Artificial Sweeteners and
ObesityCurr. Gastroenterol. Rep.19(12)646410.1007/s11894-017-0602-9
Pereira, M. J., Thombare, K., Sarsenbayeva, A., Kamble, P. G.,
Almby, K., Lundqvist, M. & Eriksson, J. W. (2020). Direct effects of
glucagon on glucose uptake and lipolysis in human adipocytes. Mol. Cell
Endocrinol., 503, 110696. DOI:
10.1016/j.mce.2019.110696
M. J. Pereira
K. Thombare
A. Sarsenbayeva
P. G. Kamble
K. Almby
M. Lundqvist
J. W. Eriksson
2020Direct effects of glucagon on glucose uptake and lipolysis in
human adipocytesMol. Cell Endocrinol.50311069610.1016/j.mce.2019.110696
Perera, N. D. & Turner, B. J. (2016). AMPK Signalling and
Defective Energy Metabolism in Amyotrophic Lateral Sclerosis.
Neurochemical Research, 41(3), 544-553. DOI:
10.1007/s11064-015-1665-3
N. D. Perera
B. J. Turner
2016AMPK Signalling and Defective Energy Metabolism in Amyotrophic
Lateral SclerosisNeurochemical Research41(3)54455310.1007/s11064-015-1665-3
Pietrocola, F., Demont, Y., Castoldi, F., Enot, D., Durand, S.,
Semeraro, M., Baracco, E. E., Pol, J., Bravo-San Pedro, J. M., Bordenave, C.,
Levesque, S., Humeau, J., Chery, A., Métivier, D., Madeo, F., Maiuri, M. C.
& Kroemer, G. (2017). Metabolic effects of fasting on human and mouse blood
in vivo. Autophagy, 13(3),
567-578. DOI: 10.1080/15548627.2016.1271513
F. Pietrocola
Y. Demont
F. Castoldi
D. Enot
S. Durand
M. Semeraro
E. E. Baracco
J. Pol
J. M. Bravo-San Pedro
C. Bordenave
S. Levesque
J. Humeau
A. Chery
D. Métivier
F. Madeo
M. C. Maiuri
G. Kroemer
2017Metabolic effects of fasting on human and mouse blood in
vivoAutophagy,13(3)56757810.1080/15548627.2016.1271513
Possik, E., Madiraju, S. R. M. & Prentki, M. (2017).
Glycerol-3-phosphate phosphatase/PGP: Role in intermediary metabolism and target
for cardiometabolic diseases. Biochimie, 143,
18-28. DOI: 10.1016/j.biochi.2017.08.001
E. Possik
S. R. M. Madiraju
M. Prentki
2017Glycerol-3-phosphate phosphatase/PGP: Role in intermediary
metabolism and target for cardiometabolic diseasesBiochimie143182810.1016/j.biochi.2017.08.001
Priyadarshini, E. & Anuradha, C. V. (2017). Glucocorticoid
Antagonism Reduces Insulin Resistance and Associated Lipid Abnormalities in
High-Fructose-Fed Mice. Can. J. Diabetes, 41(1),
41-51. DOI: 10.1016/j.jcjd.2016.06.003
E. Priyadarshini
C. V. Anuradha
2017Glucocorticoid Antagonism Reduces Insulin Resistance and
Associated Lipid Abnormalities in High-Fructose-Fed MiceCan. J. Diabetes41(1)415110.1016/j.jcjd.2016.06.003
Quiroga, A. D. & Lehner, R. (2012). Liver triacylglycerol
lipases. Biochim. Biophys. Acta, 1821(5), 762-769.
DOI:10.1016/j.bbalip.2011.09.007
A. D. Quiroga
R. Lehner
2012Liver triacylglycerol lipasesBiochim. Biophys. Acta1821(5)76276910.1016/j.bbalip.2011.09.007
Quiroga, A. D. & Lehner, R. (2018). Pharmacological intervention
of liver triacylglycerol lipolysis: The good, the bad and the ugly.
Biochem. Pharmacol., 155, 233-241. DOI:
10.1016/j.bcp.2018.07.005
A. D. Quiroga
R. Lehner
2018Pharmacological intervention of liver triacylglycerol lipolysis:
The good, the bad and the uglyBiochem. Pharmacol.15523324110.1016/j.bcp.2018.07.005
Rambold, A. S., Cohen, S. & Lippincott-Schwartz, J. (2015).
Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis,
autophagy, and mitochondrial fusion dynamics. Dev. Cell,
32(6), 678-692. DOI:
10.1016/j.devcel.2015.01.029
A. S. Rambold
S. Cohen
J. Lippincott-Schwartz
2015Fatty acid trafficking in starved cells: regulation by lipid
droplet lipolysis, autophagy, and mitochondrial fusion
dynamicsDev. Cell32(6)67869210.1016/j.devcel.2015.01.029
Randle, P. J., Garland, P. B., Hales, C. N. & Newsholme, E. A.
(1963). The glucose fatty-acid cycle. Its role in insulin sensitivity and the
metabolic disturbances of diabetes mellitus. Lancet,
1(7285), 785-789. DOI:
10.1016/s0140-6736(63)91500-9
P. J. Randle
P. B. Garland
C. N. Hales
E. A. Newsholme
1963The glucose fatty-acid cycle. Its role in insulin sensitivity and
the metabolic disturbances of diabetes mellitusLancet1(7285)78578910.1016/s0140-6736(63)91500-9
Rodríguez Delgado, J. (2017). Azúcares... ¿los malos de la dieta?
Pediatría Atención Primaria, 19,
69-75.
J. Rodríguez Delgado
2017Azúcares... ¿los malos de la dieta?Pediatría Atención Primaria196975
Roglans, N., Sanguino, E., Peris, C., Alegret, M., Vázquez, M.,
Adzet, T., Díaz, C., Hernández, G., Laguna, J. C. & Sánchez, R. M. (2002).
Atorvastatin treatment induced peroxisome proliferator-activated receptor alpha
expression and decreased plasma nonesterified fatty acids and liver triglyceride
in fructose-fed rats. J. Pharmacol. Exp. Ther.,
302(1), 232-239. DOI: 10.1124/jpet.302.1.232
N. Roglans
E. Sanguino
C. Peris
M. Alegret
M. Vázquez
T. Adzet
C. Díaz
G. Hernández
J. C. Laguna
R. M. Sánchez
2002Atorvastatin treatment induced peroxisome proliferator-activated
receptor alpha expression and decreased plasma nonesterified fatty acids and
liver triglyceride in fructose-fed ratsJ. Pharmacol. Exp. Ther.302(1)23223910.1124/jpet.302.1.232
Roglans, N., Vila, L., Farre, M., Alegret, M., Sánchez, R. M.,
Vázquez-Carrera, M. & Laguna, J. C. (2007). Impairment of hepatic Stat-3
activation and reduction of PPARalpha activity in fructose-fed rats.
Hepatology, 45(3), 778-788. DOI:
10.1002/hep.21499
N. Roglans
L. Vila
M. Farre
M. Alegret
R. M. Sánchez
M. Vázquez-Carrera
J. C. Laguna
2007Impairment of hepatic Stat-3 activation and reduction of
PPARalpha activity in fructose-fed ratsHepatology45(3)77878810.1002/hep.21499
Samuel, V. T. (2011). Fructose induced lipogenesis: from sugar to
fat to insulin resistance. Trends Endocrinol. Metab.,
22(2), 60-65. DOI: 10.1016/j.tem.2010.10.003
V. T. Samuel
2011Fructose induced lipogenesis: from sugar to fat to insulin
resistanceTrends Endocrinol. Metab.22(2)606510.1016/j.tem.2010.10.003
Sánchez-Gurmaches, J., Tang, Y., Jespersen, N. Z., Wallace, M.,
Martinez Calejman, C., Gujja, S., Li, H., Edwards, Y. J. K., Wolfrum, C.,
Metallo, C. M., Nielsen, S., Scheele, C. & Guertin, D. A. (2018). Brown Fat
AKT2 Is a Cold-Induced Kinase that Stimulates ChREBP-Mediated De Novo
Lipogenesis to Optimize Fuel Storage and Thermogenesis. Cell
Metab., 27(1), 195-209 e196. DOI:
10.1016/j.cmet.2017.10.008
J. Sánchez-Gurmaches
Y. Tang
N. Z. Jespersen
M. Wallace
C. Martinez Calejman
S. Gujja
H. Li
Y. J. K. Edwards
C. Wolfrum
C. M. Metallo
S. Nielsen
C. Scheele
D. A. Guertin
2018Brown Fat AKT2 Is a Cold-Induced Kinase that Stimulates
ChREBP-Mediated De Novo Lipogenesis to Optimize Fuel Storage and
ThermogenesisCell Metab.27(1)195209e19610.1016/j.cmet.2017.10.008
Sievenpiper, J. L., de Souza, R. J., Cozma, A. I., Chiavaroli, L.,
Ha, V. & Mirrahimi, A. (2014). Fructose vs. glucose and
metabolism: do the metabolic differences matter? Curr. Opin.
Lipidol., 25(1), 8-19. DOI:
10.1097/MOL.0000000000000042
J. L. Sievenpiper
R. J. de Souza
A. I. Cozma
L. Chiavaroli
V. Ha
A. Mirrahimi
2014Fructose vs. glucose and metabolism: do the metabolic differences
matter?Curr. Opin. Lipidol.25(1)81910.1097/MOL.0000000000000042
Smith, K. B. & Smith, M. S. (2016). Obesity Statistics.
Primare, 43(1), 121-135, ix. DOI:
10.1016/j.pop.2015.10.001
K. B. Smith
M. S. Smith
2016Obesity StatisticsPrimare43(1)121135ix10.1016/j.pop.2015.10.001
Song, Z., Xiaoli, A. M. & Yang, F. (2018). Regulation and
Metabolic Significance of De Novo Lipogenesis in Adipose Tissues.
Nutrients, 10(10). DOI:
10.3390/nu10101383
Z. Song
A. M. Xiaoli
F. Yang
2018Regulation and Metabolic Significance of De Novo Lipogenesis in
Adipose TissuesNutrients10(10)10.3390/nu10101383
Stanhope, K. L. (2016). Sugar consumption, metabolic disease and
obesity: The state of the controversy. Crit. Rev. Clin. Lab.
Sci., 53(1), 52-67. DOI:
10.3109/10408363.2015.1084990
K. L. Stanhope
2016Sugar consumption, metabolic disease and obesity: The state of
the controversyCrit. Rev. Clin. Lab. Sci.53(1)526710.3109/10408363.2015.1084990
Summermatter, S., Marcelino, H., Arsenijevic, D., Buchala, A.,
Aprikian, O., Assimacopoulos-Jeannet, F., Seydoux, J., Montani, J. P., Solinas,
G. & Dulloo, A. G. (2009). Adipose Tissue Plasticity During Catch-Up Fat
Driven by Thrifty Metabolism Diabetes, 58(10),
2228-2237. DOI: 10.2337/db08-1793
S. Summermatter
H. Marcelino
D. Arsenijevic
A. Buchala
O. Aprikian
F. Assimacopoulos-Jeannet
J. Seydoux
J. P. Montani
G. Solinas
A. G. Dulloo
2009Adipose Tissue Plasticity During Catch-Up Fat Driven by Thrifty
MetabolismDiabetes58(10)2228223710.2337/db08-1793
Ter Horst, K. W. & Serlie, M. J. (2017). Fructose Consumption,
Lipogenesis, and Non-Alcoholic Fatty Liver Disease.
Nutrients, 9(9). DOI:
10.3390/nu9090981
K. W. Ter Horst
M. J. Serlie
2017Fructose Consumption, Lipogenesis, and Non-Alcoholic Fatty Liver
DiseaseNutrients9(9)10.3390/nu9090981
Verges, B. (2018). mTOR and Cardiovascular Diseases: Diabetes
Mellitus. Transplantation, 102(2S Suppl 1),
S47-S49. DOI: 10.1097/TP.0000000000001722
B. Verges
2018mTOR and Cardiovascular Diseases: Diabetes
MellitusTransplantation102(2SSuppl1)S47S4910.1097/TP.0000000000001722
Yoon, M-S. (2017). The Role of Mammalian Target of Rapamycin (mTOR)
in Insulin Signaling. Nutrients 2017, 9, 1176.
DOI:10.3390/nu9111176
M-S. Yoon
2017The Role of Mammalian Target of Rapamycin (mTOR) in Insulin
SignalingNutrients201791176117610.3390/nu9111176
World Health Organization. World Health Statistics (2018):
Monitoring Health for the SDGs., 2018.
World Health Organization
World Health Statistics (2018): Monitoring Health for the SDGs2018
TIP REVISTA ESPECIALIZADA EN CIENCIAS QUÍMICO-BIOLÓGICAS, Volumen 26, 2023, es una publicación editada por la Universidad Nacional Autónoma de México, Ciudad Universitaria, Deleg. Coyoacán, C.P. 04510, Ciudad de México, México, a través de la Facultad de Estudios Superiores Zaragoza, Campus I, Av. Guelatao # 66, Col. Ejército de Oriente, Deleg. Iztapalapa, C.P. 09230, Ciudad de México, México, Teléfono: 55.56.23.05.27, http://tip.zaragoza.unam.mx, Correo electrónico revistatip@yahoo.com, Editor responsable: Dra. Martha Asunción Sánchez Rodríguez, Certificado de Reserva de Derechos al Uso Exclusivo del Título No. 04-2014-062612263300-203, ISSN impreso: 1405-888X, ISSN electrónico: 2395-8723, otorgados por el Instituto Nacional del Derecho de Autor, Responsable de la última actualización de este número Claudia Ahumada Ballesteros, Facultad de Estudios Superiores Zaragoza, Av. Guelatao # 66, Col. Ejército de Oriente, Deleg. Iztapalapa, C.P. 09230, Ciudad de México, México, fecha de la última modificación, 27 de febrero de 2025.
Esta página puede ser reproducida con fines no lucrativos, siempre y cuando no se mutile, se cite la fuente completa y su dirección electrónica. De otra forma requiere permiso previo de la institución.


